Shanghai, China – June 10, 2025 – As China advances its national strategy to foster pharmaceutical innovation and drive high-quality industry growth, the country’s biopharma ecosystem is experiencing unprecedented acceleration. Domestic innovators are making remarkable strides in global markets, yet the industry still lacks a robust, internationally benchmarked framework to objectively evaluate clinically meaningful innovations within China’s distinctive healthcare landscape.
Therefore, SAI MedPartners (SAI), Shanghai Health Development Research Center (SHDRC), and China Pharmaceutical Innovation and Research Development Association (PhIRDA) have jointly launched the development of the China Pharmaceutical Innovation and Invention Index (China PIII). This initiative aims to establish a comprehensive framework for evaluating the innovative performance of the domestic pharmaceutical enterprises, spanning areas such as novel drug R&D, technology translation, and commercialization readiness. The index is designed to further support policy improvement, optimize resource allocation, and guide long-term industry development strategies.
Leveraging Global Expertise to Develop China PIII
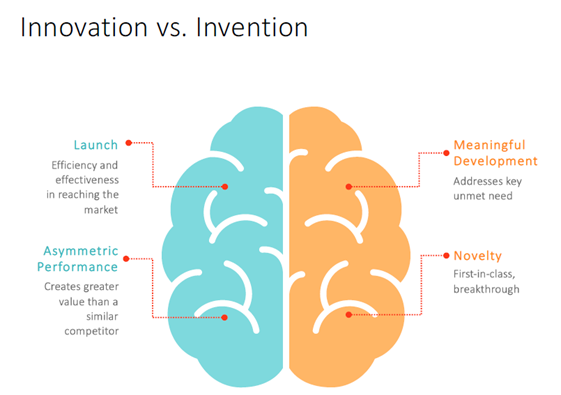
For the past 14 years, IDEA Pharma, a subsidiary of SAI MedPartners, has published the globally recognized Pharmaceutical Innovation and Invention Index (PIII), which evaluates pharmaceutical companies across both “innovation” and “invention” dimensions. The PIII framework has become an indispensable reference for evaluating innovative performance across the international biopharma sector.
Rooted in global methodologies, yet tailored for China’s unique context, the China PIII strategically aligns with local policy priorities, healthcare delivery models, and industrial innovation patterns – delivering an authoritative evaluation standard that bridges worldwide expertise with localized implementation.
High-level Stakeholder Panel Successfully Convened
On June 10, 2025, SAI Asia co-hosted a high-level stakeholder panel meeting with SHDRC and PhIRDA on Building China’s Pharmaceutical Innovation Evaluation System, with the aim to further refine and validate the China PIII framework. The meeting brought together key stakeholders from regulatory agencies, industry innovation associations, top-tier hospital executives, academia, and C-suite executives from leading domestic pharmaceutical companies.
Panel discussions focused on the China PIII’s objectives, application scenarios, framework optimization, key metrics adjustment, and localization strategies, ensuring its relevance in China’s rapidly evolving healthcare landscape. This high-level convening established critical consensus around developing an index that combines scientific validity with strategic foresight, while commanding widespread credibility across China’s pharmaceutical ecosystem.

Leadership Perspectives on the Value of the China PIII
Prof. Jin Chunlin, President of SHDRC, emphasized that while national policies strongly encourage pharmaceutical innovation, a scientific and standardized evaluation system is essential to properly define, assess, and drive innovation. He highlighted the need to harmonize global benchmarks with China-specific contexts, accommodate diverse innovation maturity levels, and ensure the framework remains both rigorous in methodology and actionable in practice.
Mr. Song Ruilin, Executive President of PhIRDA, noted that various healthcare stakeholders – including payers, regulators, and healthcare providers – currently lack consensus in defining “innovation” in China. The China PIII aims to bridge this gap by establishing an independent, evidence-based assessment framework that not only aligns domestic perspectives but also enhances global visibility of China’s innovation milestones.
SAI’s Commitment to Supporting China’s Innovation Advancement
Celia Deng, President of SAI Asia, presented the global foundation and industry impact of the PIII framework. She reaffirmed three key commitments: 1) fostering open partnerships with Chinese stakeholders, 2) facilitating comprehensive knowledge exchange, and 3) providing expert support to develop China’s premier innovation evaluation system – one that meets the highest standards of scientific validity while maintaining global benchmarking relevance.
Ryan Li, Head of Market Access and RWE at SAI Asia, delivered a keynote presentation outlining the initial framework and localization strategy for the China PIII, detailing its two-dimensional evaluation model, synthesizing critical industry feedback, and outlining actionable implementation plans – all of which stimulated an engaged, solutions-oriented discussion among attending experts from across the pharmaceutical ecosystem.
Broad Industry Consensus on the Strategic Importance of China PIII
During the roundtable discussion, participating experts reached a strong consensus on the significance of developing the China Pharmaceutical Innovation Index. They offered comprehensive recommendations to enhance scientific robustness, practical implementation, and policy alignment, recognizing its potential to both guide domestic innovation growth and facilitate global integration.
The collaborative development of the China PIII marks a critical step in establishing a world-class innovation evaluation system that supports China’s continued emergence as a global leader in pharmaceutical innovation.

About Shanghai Health Development Research Center (SHDRC)
The Shanghai Health Development Research Center is a leading think tank and policy research institution for healthcare reform and healthcare security development, dedicated to advancing healthcare policy, industry research, and healthcare innovation strategies.
上海市卫生和健康发展研究中心(上海市医学科学技术情报研究所)
About China Pharmaceutical Innovation and Research Development Association (PhIRDA)
PhIRDA is a national industry association focused on promoting pharmaceutical innovation, regulatory policy development, and international collaboration within China’s life sciences sector.
China Pharmaceutical Industry Research Development Association (SINO-PhIRDA)


